 During these first months of 2021 I have published two papers concerning my previous research activities started at the University of Bari Aldo Moro. Among my specializations, I am expert in the synthesis and characterization of conjugated materials based on arylenethienylene structures. The first of these studies describes the preparation and characterization of diketopyrrolopyrrole (DPP) and thiophene -based oligomers, decorated with -SAc groups. This work is the natural evolution of my PhD thesis, that was centered on the preparation of thiol oligoarylenes for molecular electronics.
During these first months of 2021 I have published two papers concerning my previous research activities started at the University of Bari Aldo Moro. Among my specializations, I am expert in the synthesis and characterization of conjugated materials based on arylenethienylene structures. The first of these studies describes the preparation and characterization of diketopyrrolopyrrole (DPP) and thiophene -based oligomers, decorated with -SAc groups. This work is the natural evolution of my PhD thesis, that was centered on the preparation of thiol oligoarylenes for molecular electronics.
During my PhD studies, the group of Alan Heeger published a paper on Nature Materials describing how aliphatic thiols additives were able to improve the performances of polymeric bulk-heterojunction solar cells. Later on, it was explained that the aliphatic thiol was able to dissolve the fullerene counterpart. This was the period in which I decided to synthesize thiol decorated oligo- and polythiophenes and study their blend forming properties mixing them with PCBM. It took a long time to study these systems, as they were introduced in BHJ solar cells as third components, and understanding the behavior of ternary blends is not easy. Resolutive was my visit at the Imperial College in 2015, in the group of Prof. Natalie Stingelin, were I performed in collaboration with her group differential scanning calorimetry studies on the melting properties of our blends. This was truly helpful to understand the physical chemistry going on there. The result was a paper full of experimental evidences, published in Chemistry of Materials in 2018.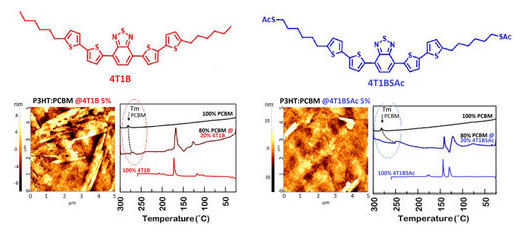 Graphical abstract of the article “Designing Small Molecules as Ternary Energy-Cascade Additives for Polymer:Fullerene Solar Cell Blends” by A. Punzi, A. Operamolla, O. Hassan Omar, F. Brunetti, A. D. Scaccabarozzi, G. M. Farinola and N. Stingelin, published in Chem. Mater. 2018, doi: 10.1021/acs.chemmater.8b00675
Graphical abstract of the article “Designing Small Molecules as Ternary Energy-Cascade Additives for Polymer:Fullerene Solar Cell Blends” by A. Punzi, A. Operamolla, O. Hassan Omar, F. Brunetti, A. D. Scaccabarozzi, G. M. Farinola and N. Stingelin, published in Chem. Mater. 2018, doi: 10.1021/acs.chemmater.8b00675
This year, we have published the natural progress of that paper. In the aim of preparing novel additives for BHJ solar cells, we observed that the S-Acyl group decorating DPP-oligomers was able to induce a huge red-shift of absorbed solid-state wavelength, which correspond to enhanced formation of J-aggregates. This behavior is peculiar, and can be replicated also in solid state nanoparticles. The results were interesting and deserved a publication in the journal RSC Advances, as a Gold Open Access paper.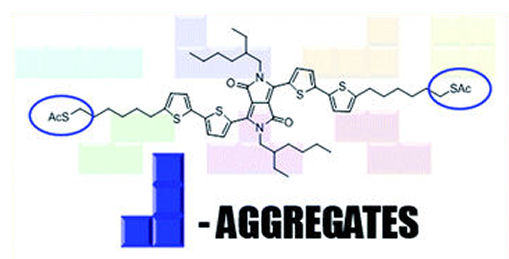 Graphical abstract of the article “Peripherical thioester functionalization induces J-aggregation in bithiophene-DPP films and nanoparticles” by A. Punzi, D. Blasi, A. Operamolla, R. Comparelli, G. Palazzo and G. M. Farinola, published in RSC Adv. 2021, doi: 10.1039/D1RA01253C
Graphical abstract of the article “Peripherical thioester functionalization induces J-aggregation in bithiophene-DPP films and nanoparticles” by A. Punzi, D. Blasi, A. Operamolla, R. Comparelli, G. Palazzo and G. M. Farinola, published in RSC Adv. 2021, doi: 10.1039/D1RA01253C
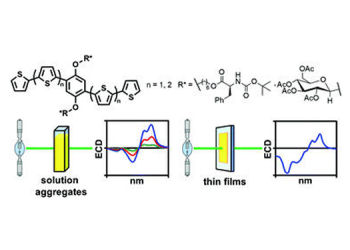
The second paper is pertinent to another class of thiophenearylene – based materials, on which we used the alkoxy substituents to bind enantiomerically pure small biomolecules. More specifically, we are able to decorate the conjugated framework with D-glucopyranose units via beta-glycosides and with tBOC-protected L-phenylalanine via ester bond formation. These materials and their films can be studied by electronic circular dicroism technique (ECD) to get useful insides on the mechanism of their solid-state aggregation. This study is published in the RSC journal New Journal of Chemistry.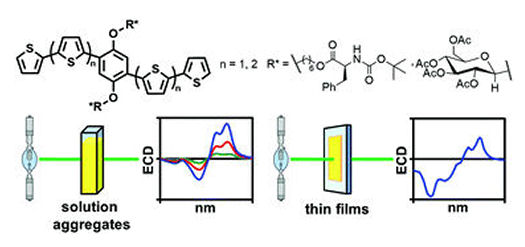 Graphical abstract of the article “Impact of chirality on the aggregation modes of l-phenylalanine- and d-glucose-decorated phenylene–thiophene oligomers” by O. Hassan Omar, M. Falcone, A. Operamolla and G. Albano, published in New J. Chem. 2021, doi: 10.1039/D1NJ02125G
Graphical abstract of the article “Impact of chirality on the aggregation modes of l-phenylalanine- and d-glucose-decorated phenylene–thiophene oligomers” by O. Hassan Omar, M. Falcone, A. Operamolla and G. Albano, published in New J. Chem. 2021, doi: 10.1039/D1NJ02125G
Copyrights of this article belong to Alessandra Operamolla. All rights reserved.
